Obesity has become a global epidemic, with the number of individuals affected nearly tripling in the past 50 years. This alarming rise in obesity rates has led to a significant public health crisis, as it is closely associated with numerous debilitating conditions such as diabetes, heart disease, and cancer. While the detrimental effects of obesity on overall health are well-known, the underlying mechanisms remain elusive. However, a recent study has shed light on how obesity directly affects mitochondria, the powerhouses of the cell responsible for energy production.
Mitochondria play a vital role in generating energy within cells. However, individuals with obesity often experience impaired mitochondrial function, leading to a reduced capacity for burning fat. To investigate this phenomenon further, an international team of scientists conducted a study on mice fed a high-fat diet.
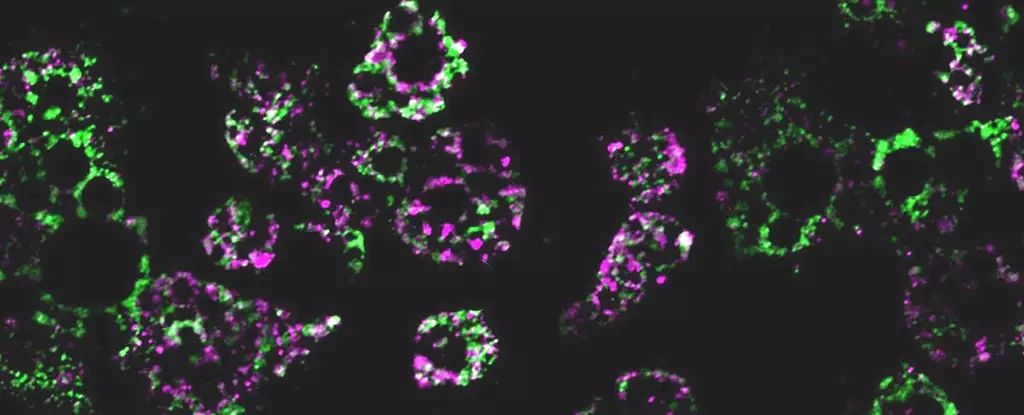
The researchers discovered that mice on a high-fat diet experienced a fragmentation of mitochondria within their fat cells. This fragmentation resulted in smaller mitochondria that were less efficient at burning fat. Furthermore, the study revealed that this process was regulated by a single gene.
When the researchers deleted the gene responsible for mitochondrial fragmentation in their test subjects, the mice were able to avoid excessive weight gain, despite being fed the same high-fat diet. This gene is critical in the metabolic transition from a healthy weight to obesity. The findings suggest that caloric overload from overeating triggers a cascade that reduces energy expenditure, exacerbating obesity. Understanding the role of this gene brings us one step closer to developing targeted therapies for weight gain and associated metabolic dysfunctions.
The researchers identified a multifunctional molecule, RalA, which controls the fragmentation of mitochondria. Normally, RalA breaks down malfunctioning mitochondria. However, the study suggests that when RalA is overactive, it interferes with the regular function of mitochondria, leading to a metabolic cascade. Chronic activation of RalA appears to play a critical role in suppressing energy expenditure in obese adipose tissue.
By understanding the mechanism behind mitochondrial fragmentation, scientists hope to develop targeted therapies that promote fat burning and address weight gain and associated metabolic dysfunctions. Deleting the gene associated with RalA in mice prevented the weight gain typically observed with a high-fat diet. While these findings are promising, further research is necessary to determine if the results translate to humans.
Although the study was conducted on mice, there are similarities between the proteins influenced by RaIA in mice and those associated with obesity and insulin resistance in humans. This suggests that the findings may be relevant to humans, opening the possibility of developing new therapies that target the RalA pathway to combat obesity.
Obesity poses a significant threat to global health, contributing to a range of debilitating conditions. Understanding how obesity affects mitochondria and discovering the role of the RalA pathway in metabolic dysfunctions brings us closer to developing targeted therapies for weight management. While further research is required, these findings provide valuable insights into the complex mechanisms underlying obesity and offer hope for more effective treatment and prevention strategies in the future.

Leave a Reply