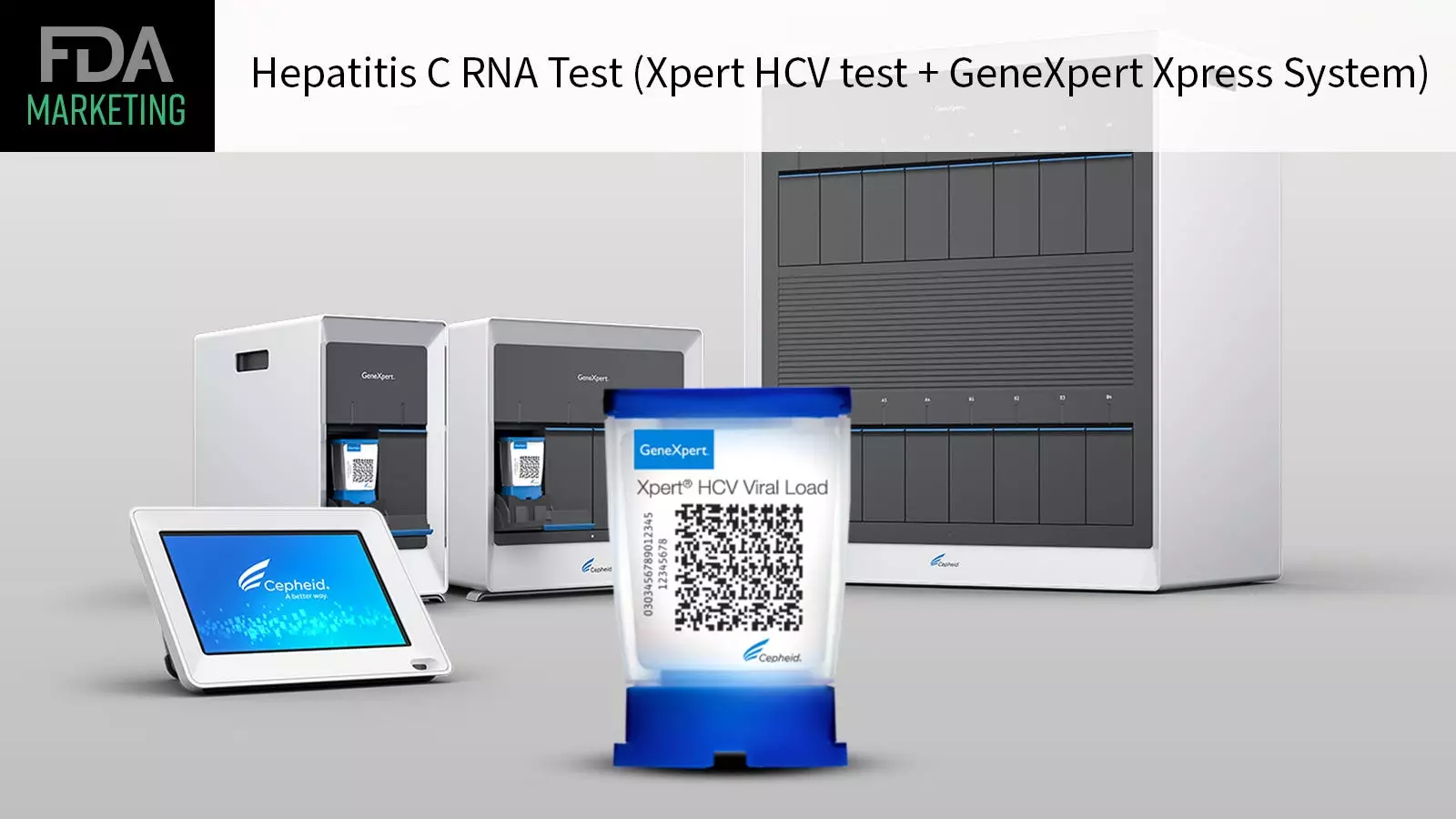
The recent FDA approval of the first point-of-care test for hepatitis C virus (HCV) marks a significant milestone in the fight against this infectious disease. The Xpert HCV test and GeneXpert Xpress System have been granted marketing authorization, allowing at-risk adults to be rapidly diagnosed and immediately connected to care if needed. This innovative test detects HCV RNA and is specifically indicated for individuals exhibiting signs or symptoms of hepatitis C. What sets this test apart is its ability to provide results on the spot, eliminating the need to send samples to a central lab. This development opens up the possibility of testing in various healthcare settings such as doctors’ offices, urgent care centers, substance use disorder treatment facilities, and emergency rooms, as long as they possess a Clinical Laboratory Improvement Amendments (CLIA) Certificate of Waiver.
The Xpert HCV test is a game-changer in the field of hepatitis C testing, allowing healthcare providers to diagnose patients quickly and efficiently. By using a fingertip blood sample, the test can deliver results within an hour, enabling providers to promptly discuss treatment options or refer patients to care in the same visit. This streamlined approach addresses the current challenges in hepatitis C testing, where multiple appointments are often required for testing, obtaining results, and further testing. This cumbersome process can result in patients with infections being lost to follow-up and not receiving a timely diagnosis or treatment.
The Impact of Early Detection
Early detection of hepatitis C is crucial in preventing disease progression and reducing the spread of the virus. While direct-acting antivirals have been available for over a decade, cure rates in the U.S. remain alarmingly low. The Centers for Disease Control and Prevention (CDC) recommended in 2020 that all adults should be screened for HCV at some point in their lives, emphasizing the importance of early detection in curbing the disease’s impact. Left untreated, chronic HCV infections can lead to severe complications such as liver cancer or liver failure. In 2022, hepatitis C was associated with over 12,000 deaths, underscoring the urgent need for improved testing and treatment strategies.
While the approval of the Xpert HCV test represents a significant advancement in hepatitis C diagnostics, challenges remain in ensuring widespread access to testing and treatment. Affordability and availability are key factors that will determine the test’s success in reaching more individuals with hepatitis C infections. The test is not intended for monitoring patients undergoing treatment for hepatitis C or for screening blood or tissue donors. Risks associated with the test include the possibility of false-positive or false-negative results, which can lead to unnecessary fears, treatment delays, and other potential consequences.
The introduction of the first point-of-care test for hepatitis C represents a critical step towards improving the diagnosis and management of this condition. By enabling rapid testing and immediate linkage to care, this innovative test has the potential to significantly impact the lives of millions of individuals affected by hepatitis C. Moving forward, it will be essential to address challenges related to affordability, availability, and ensuring accurate and timely testing for at-risk populations. Through continued innovation and collaboration, we can work towards a future where hepatitis C is no longer a leading cause of liver-related deaths.

Leave a Reply